-
EN




Cardiovascular Omics Solutions
Lipid molecules play a pivotal role in cardiac energy production, myocardial cell membrane and organelle composition, as well as in the onset and progression of cardiovascular diseases. Sphingolipids, including ceramides, sphingomyelins, and glycosphingolipids, serve as critical mediators in myocardial ischemia and inflammatory responses, contributing significantly to atherosclerosis, myocardial infarction, hypertension, and related disorders.
By integrating lipidomics as the core approach with proteomics, spatial omics, and complementary biological experiments, we provide a comprehensive solution tailored to multiple application scenarios in cardiovascular disease research and clinical management.

Product Consultation
(1)Discovery of cardiovascular biomarkers through integrated biofluid and tissue lipidomics
(2)Lipidomics-based risk stratification for cardiovascular diseases
(3)Dynamic assessment of cardiometabolic risk using lipidomic profiling.
(4)Mechanistic studies on factors affecting prognosis in cardiovascular patients.
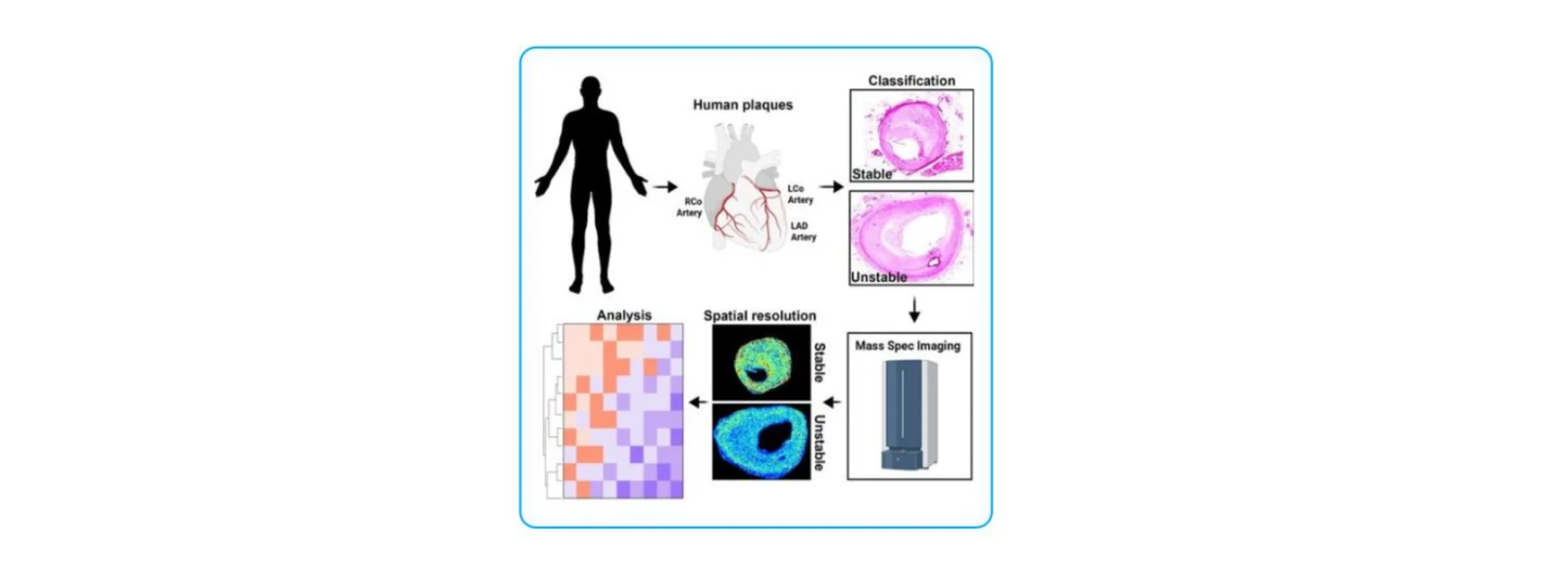
(1)Spatial Metabolomics-Based Assessment of Atherosclerotic Plaque Stability
Arterioscler Thromb Vasc Biol. 2023;43(9):1626–1635 (IF = 8.1, Q1)
Research Objective:
To characterize spatially resolved metabolic differences between stable and unstable human atherosclerotic plaques—specifically in fibrous caps and necrotic cores—and to uncover metabolic pathways associated with plaque instability.
Study Cohort:
5 cases of stable plaques and 4 cases of unstable plaques.
Key Findings:
1. Stable plaques showed enrichment of acylcarnitines, acylglycines, and long-chain fatty acid β-oxidation pathways, while unstable plaques exhibited elevated pathways related to reactive oxygen species, aromatic amino acids, and tryptophan metabolism.
2. In stable plaques, lactate was enriched in necrotic cores and pyruvate was elevated in fibrous caps. In contrast, unstable plaque caps showed accumulation of 5-hydroxytryptophan metabolites.
3. RNA sequencing revealed upregulation of genes involved in collagen metabolism and nitric oxide synthesis in stable plaques, whereas inflammatory and oxidative stress pathways were activated in unstable plaques.
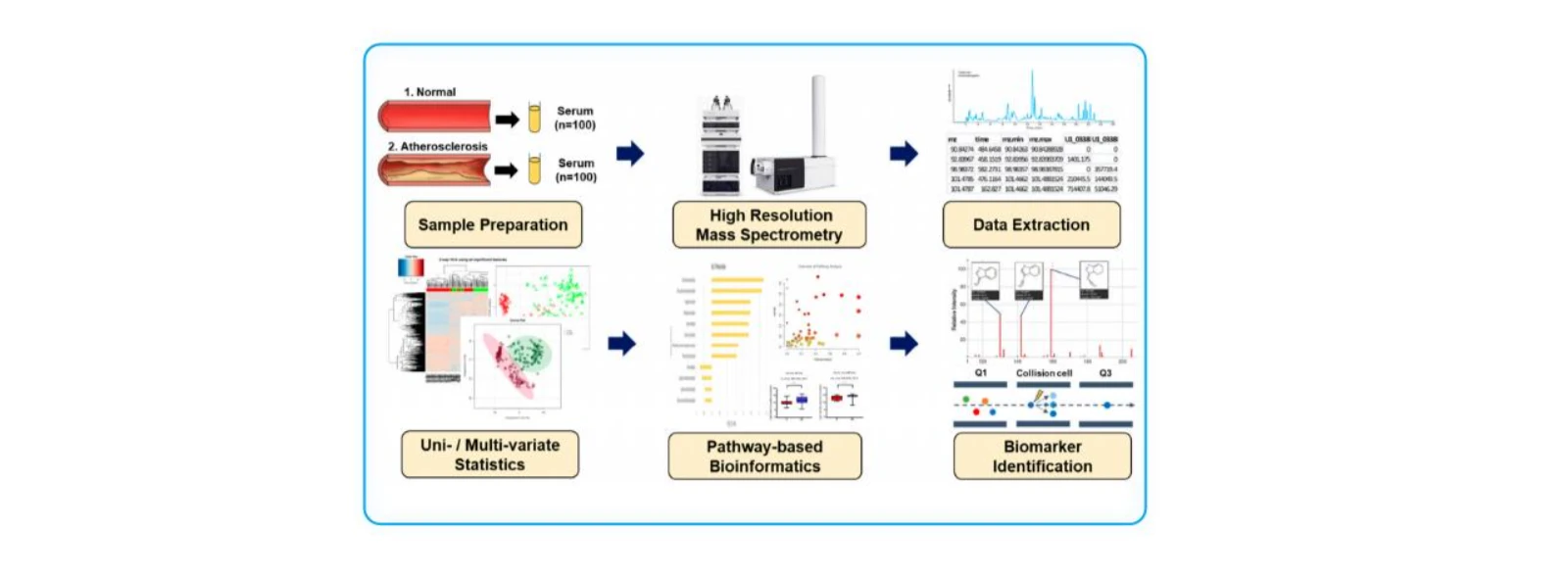
(2)Early Diagnosis of Atherosclerosis in Patients with Metabolic Disorders Using Metabolomic Biomarkers
Metabolites. 2023;13(11):1160 (IF = 4.0, Q3)
Research Objective:
To identify novel metabolomic biomarkers for the early, non-invasive diagnosis of atherosclerosis (AS) and to explore associated metabolic pathway alterations that may offer new insights into AS pathogenesis.
Study Cohort:
100 healthy controls and 100 patients with atherosclerosis.
Key Findings:
1. Cortisol, hypoxanthine, and isoleucine were identified as potential early-stage, non-invasive biomarkers for AS.
2. A random forest model combining these three markers achieved an AUC of 0.96, significantly outperforming individual biomarkers.
3. Atherosclerosis patients exhibited significant alterations in bile acid synthesis, steroid hormone metabolism, branched-chain amino acid metabolism, and purine metabolism pathways.
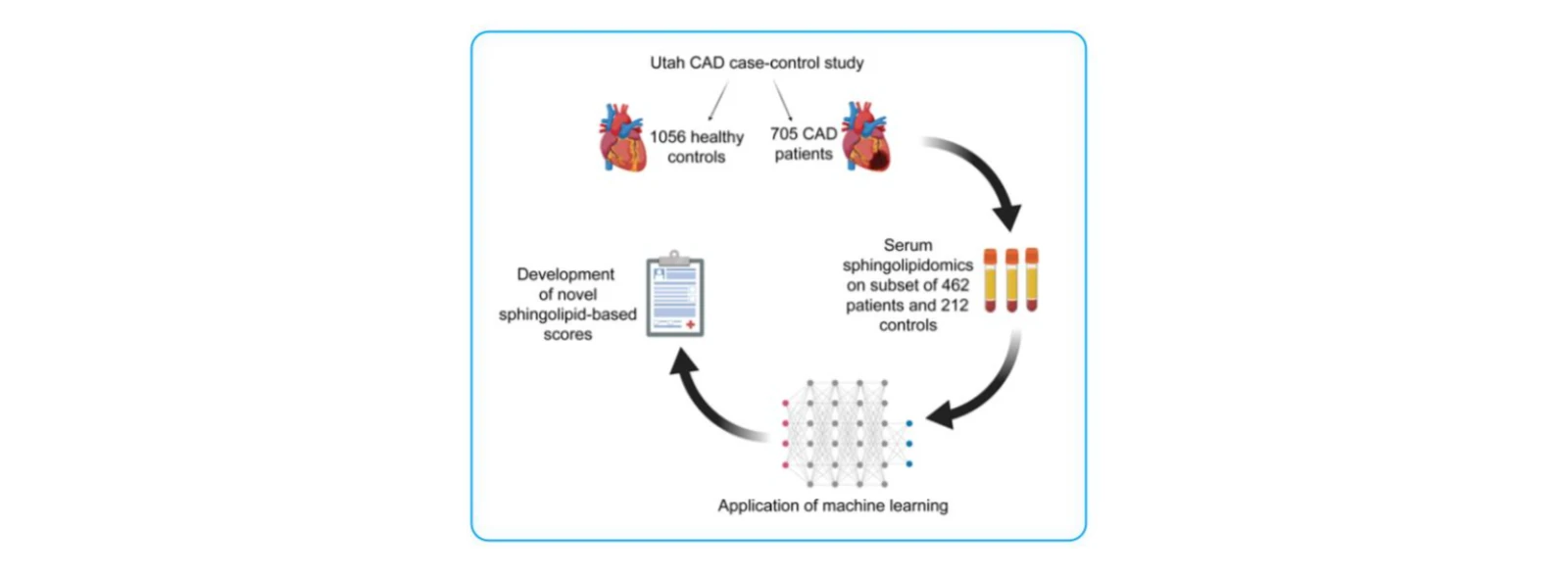
(3)Sphingolipid-Based Risk Stratification for Coronary Artery Disease
J Clin Invest. 2020;130(3):1363–1376 (IF = 14.6, Q1)
Research Objective:
To evaluate serum sphingolipids as cholesterol-independent biomarkers for coronary artery disease (CAD) and to develop a novel sphingolipid-based risk score (SIC) using machine learning, validating their predictive value in cardiovascular disease.
Study Cohort:
212 healthy controls and 462 patients with coronary artery disease.
Key Findings:
1. Thirty-two sphingolipids, including ceramides, dihydroceramides, and sphingomyelins, were significantly elevated in CAD patients.
2. A novel risk scoring system—Sphingolipid Inclusive Classifier (SIC)—was developed, incorporating lipids reflective of ceramide biosynthesis pathways. SIC demonstrated superior discriminatory power compared to traditional biomarkers and provided independent risk stratification for CAD.
(4)Dynamic Lipidomic Assessment of Cardiometabolic Risk
Nat Med. 2025;31(1):43–44 (IF = 58.7, Q1)
Research Objective:
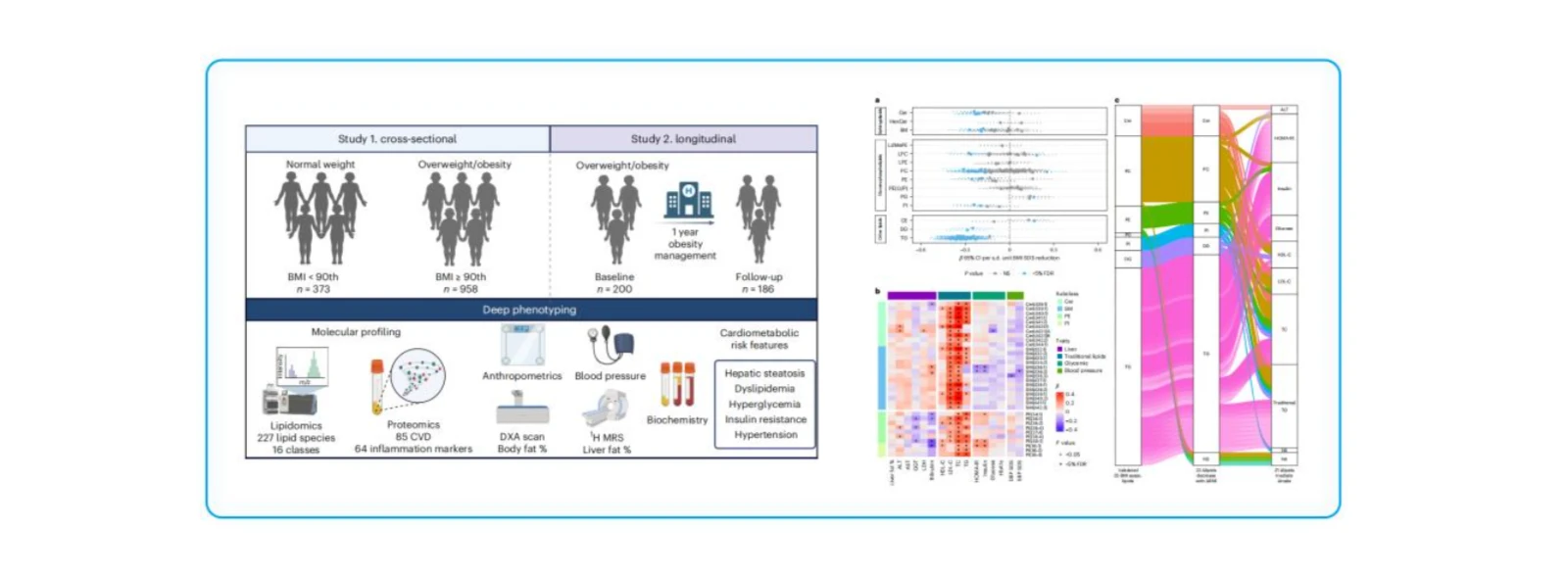
To investigate peripheral lipidomic differences between overweight/obese and normal-weight children, clarify the role of lipid classes in pediatric obesity and its cardiometabolic complications, assess the impact of clinical management on lipid profiles, and identify lipid biomarkers associated with hepatic steatosis and insulin resistance. The study also aimed to explore lipid-mediated mechanisms linking obesity to cardiometabolic risk.
Study Cohort:
Cross-sectional study: 958 overweight/obese and 373 normal-weight children.
Intervention study: 186 overweight/obese children received clinical management with baseline and follow-up lipidomic profiling.
Key Findings:
1. Distinct lipid dysregulation (↑ceramides / ↓sphingomyelins) was observed early in pediatric obesity and was strongly associated with elevated cardiometabolic risk.
2. Clinical intervention partially reversed the abnormal lipid patterns, suggesting that targeting lipid metabolism may represent a promising strategy for preventing and managing pediatric cardiovascular disease.
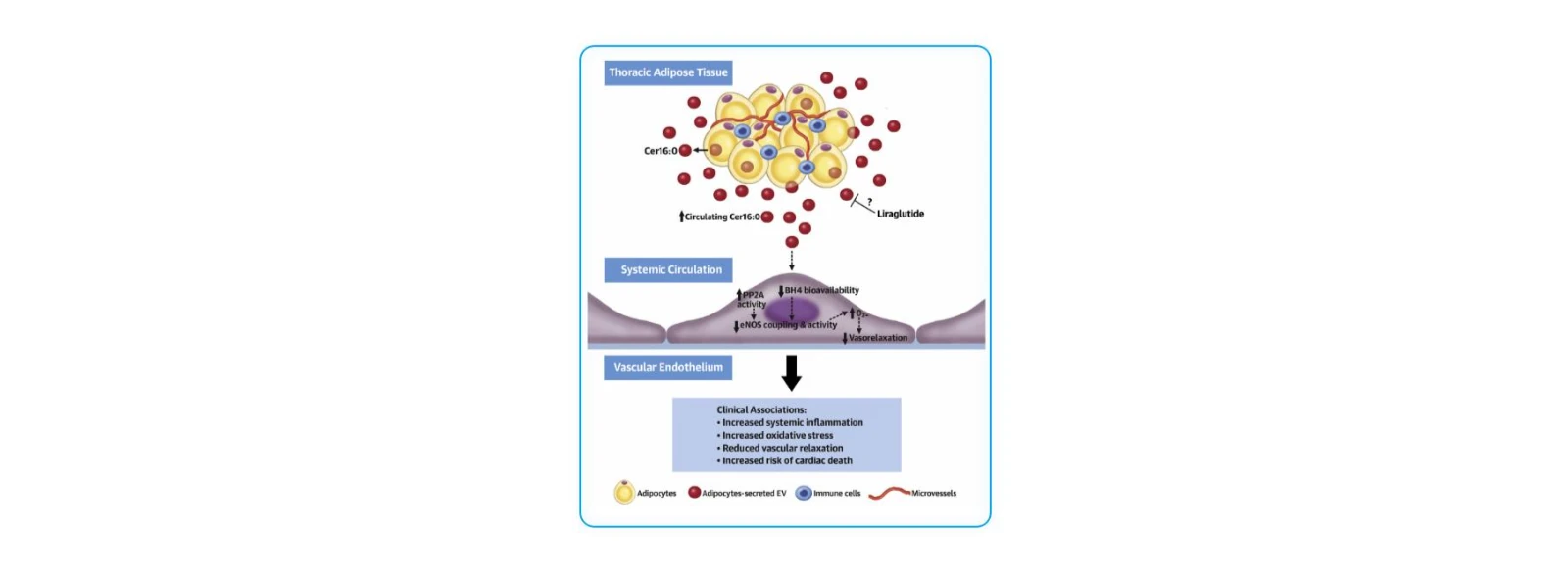
(5)Mechanistic Insights into Cardiovascular Disease: Role of Adipose-Derived Metabolites
J Am Coll Cardiol. 2021;77(20):2494–2513 (IF = 24.2, Q1)
Research Objective:
To investigate how adipose tissue metabolic dysregulation influences vascular redox signaling and cardiovascular outcomes, identify specific adipose-derived metabolites that regulate oxidative stress and endothelial function, and evaluate their potential both as prognostic biomarkers and therapeutic targets in cardiovascular disease.
Study Cohort:
48 participants (31 obese, 17 BMI-matched non-obese controls) were recruited to compare metabolomic profiles of thoracic adipose tissue (ThAT) and subcutaneous adipose tissue (ScAT).
32 obese patients were evaluated for the regulatory effect of liraglutide (a GLP-1 receptor agonist) on plasma ceramide levels.
Key Findings:
1. Ceramides secreted from adipose tissue—particularly Cer16:0—play a central role in obesity-associated vascular dysfunction.
2. Cer16:0 was identified as a potential prognostic biomarker for cardiovascular disease, and GLP-1 analogs were proposed as promising therapeutic agents for modulating ceramide-driven vascular risk.