-
EN




Geriatric Omics Solutions
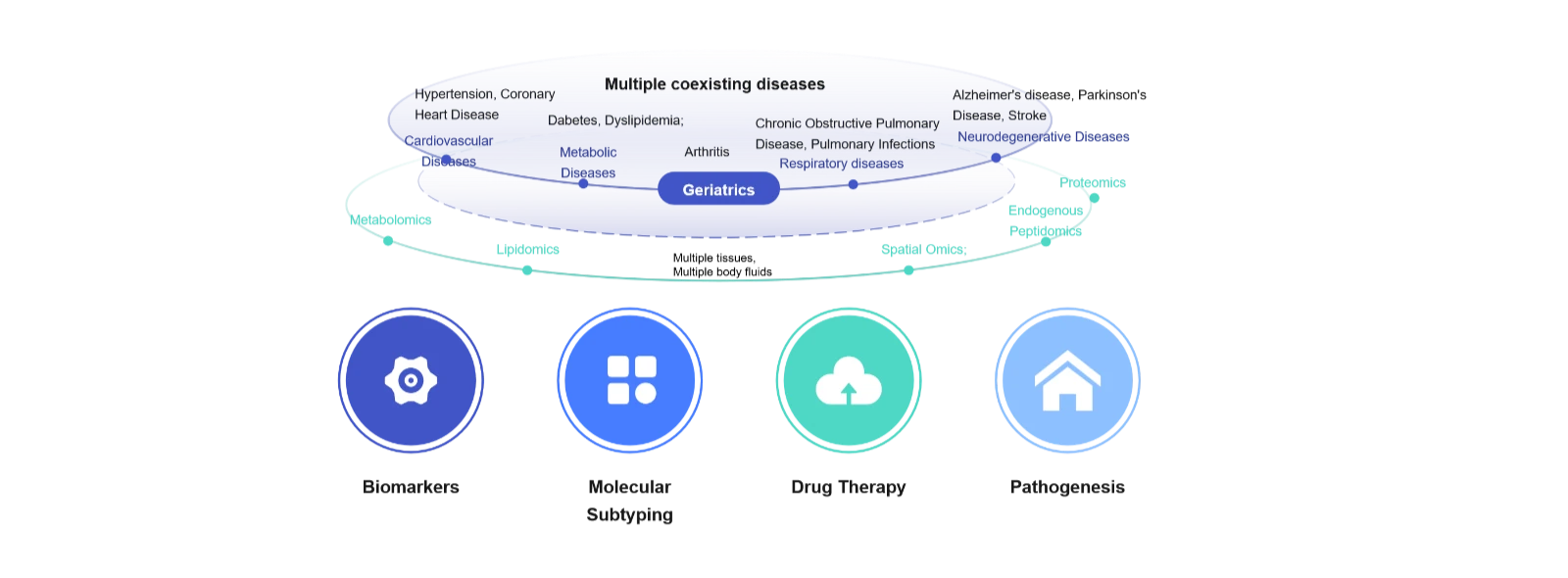
Age-related changes in energy metabolism are closely linked to the onset of chronic diseases. As individuals age, energy expenditure declines and body fat increases, contributing to a higher incidence of chronic conditions such as osteoarthritis, osteoporosis, heart failure, Alzheimer’s disease, macular degeneration, COPD, hearing loss, diabetes, atherosclerosis, and non-alcoholic fatty liver disease.
Metabolomic profiling has revealed characteristic age-related shifts in metabolic signatures—known as the “metabolic aging clock.” Key metabolites such as creatinine, serine, and phosphate decrease with age, while others including sphingomyelins, citrate, and citrulline increase. These metabolic changes not only reflect physiological decline but also offer critical insights into the biological mechanisms of aging and age-associated diseases.

Product Consultation
Common diseases and potential research directions in geriatrics
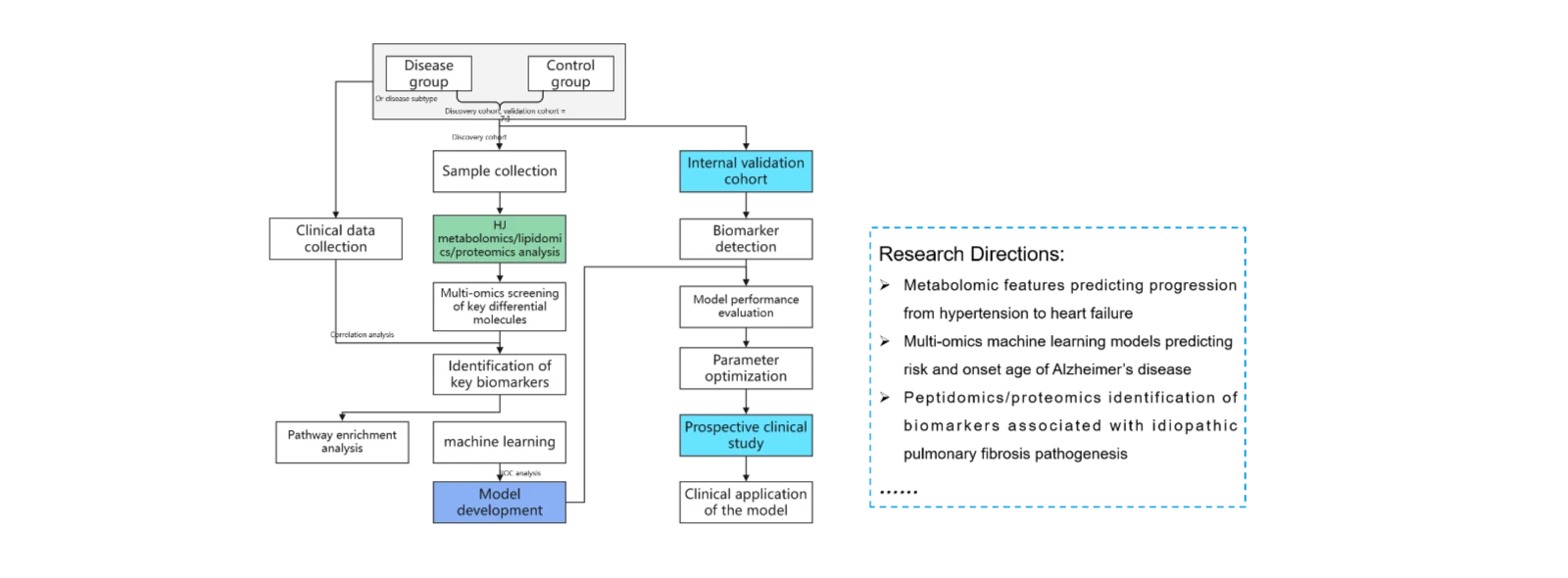
(1)Multi-omics analysis of disease biomarkers for early screening and diagnosis
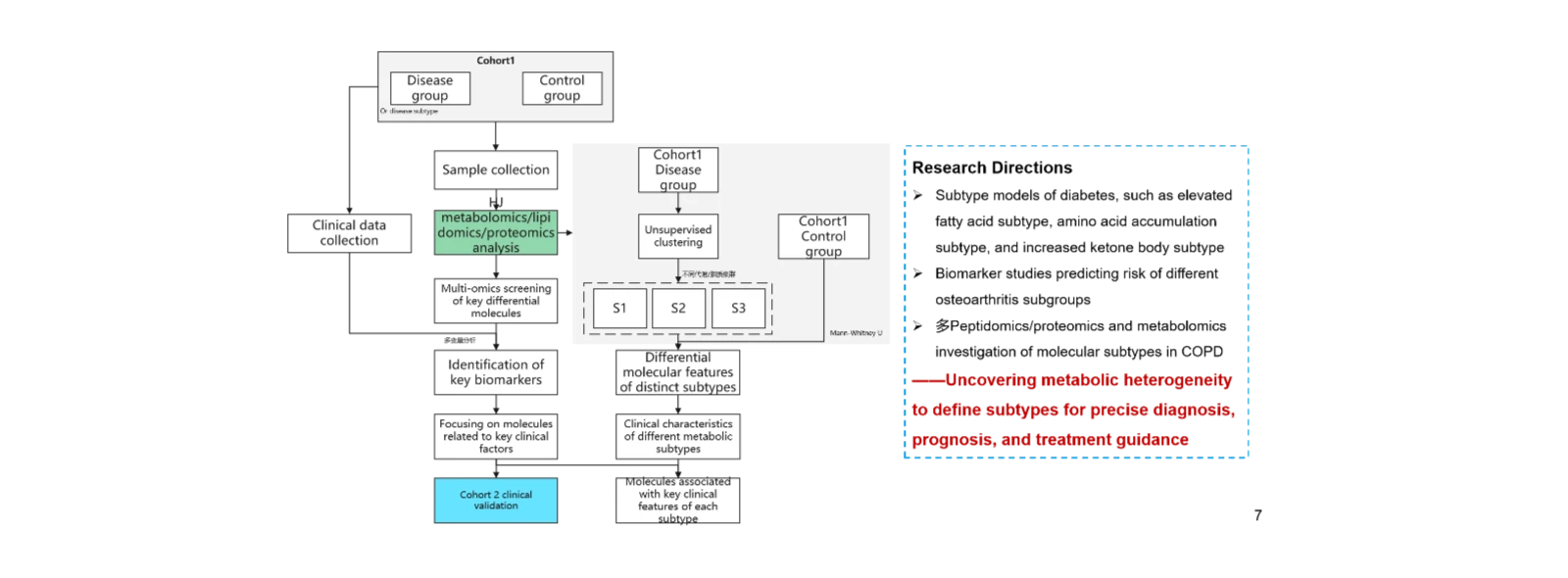
(2)Multi-omics profiling for molecular subtyping to support prognosis and precision therapy
(3)Multi-omics identification of biomarkers influencing drug response, enabling prediction and monitoring of treatment sensitivity
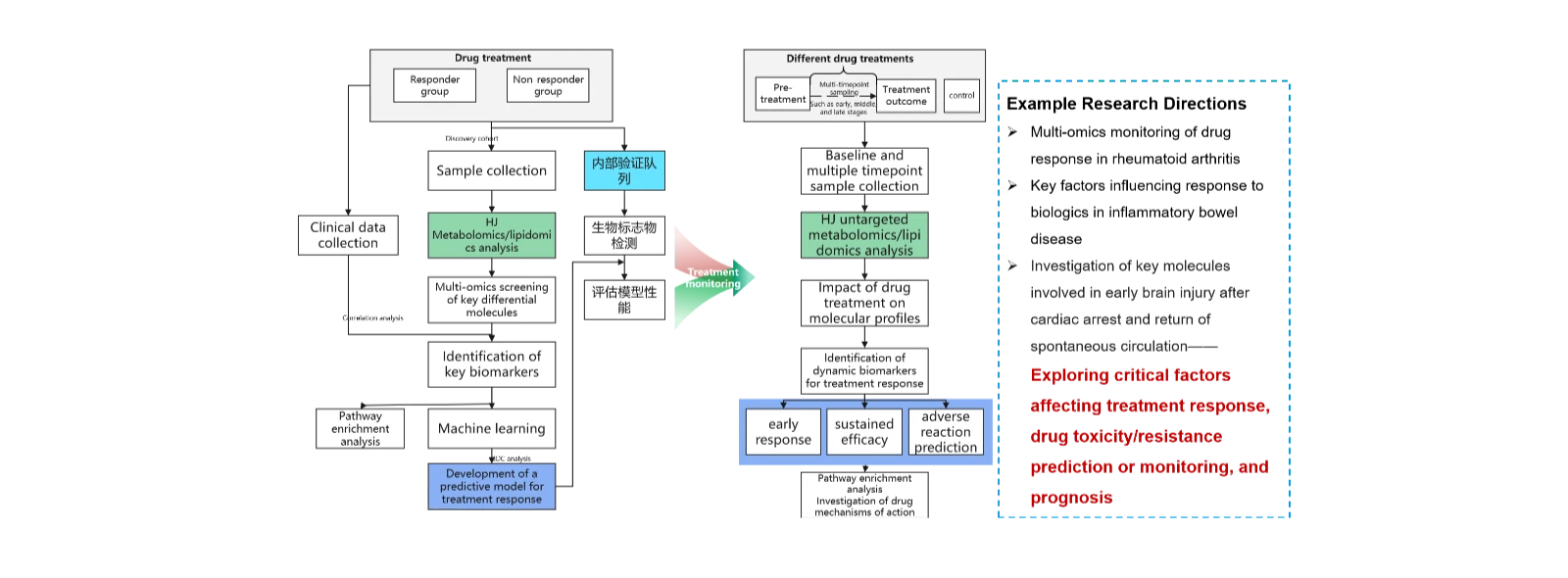
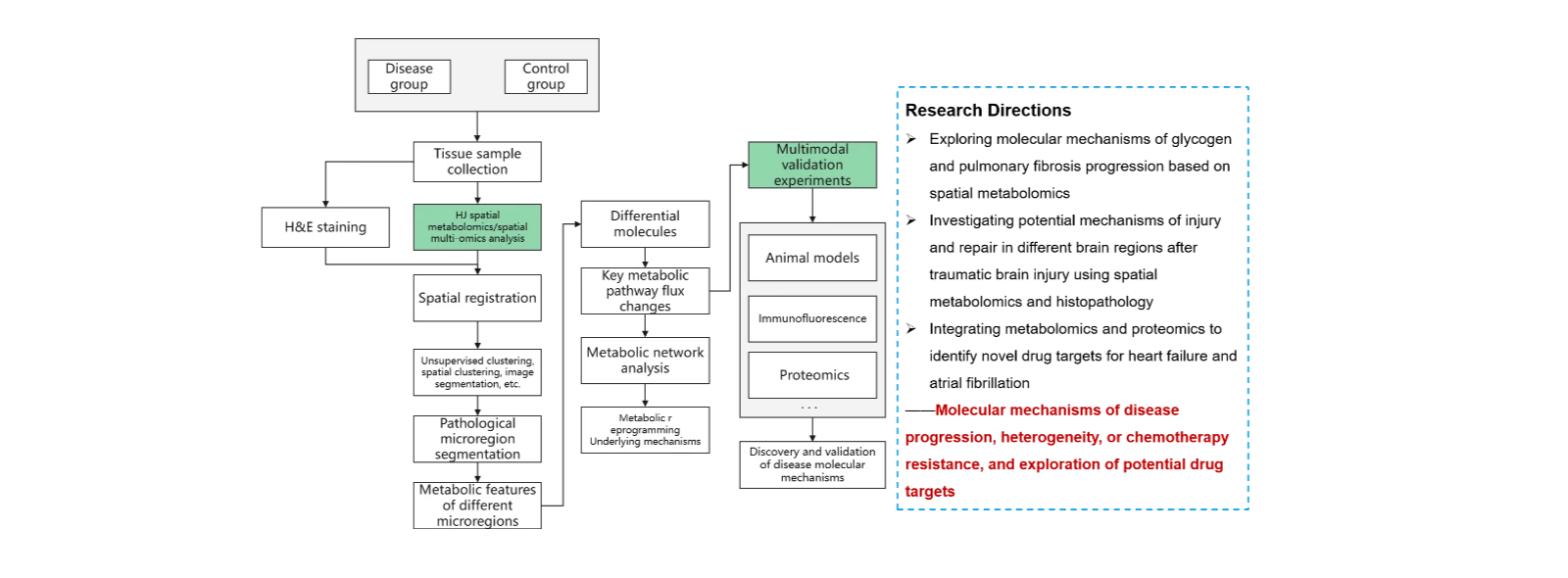
(4)Multi-omics investigation of disease molecular mechanisms.
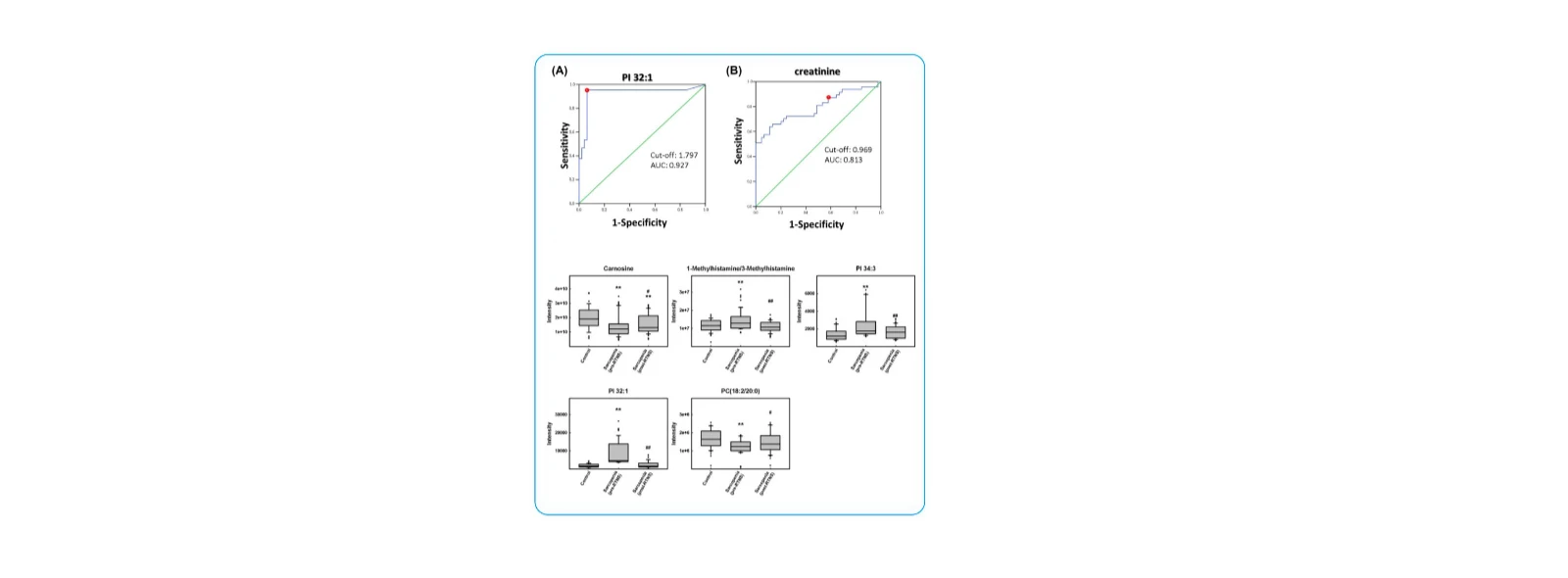
(1)Discovery of Novel Metabolomic and Lipidomic Biomarkers for Sarcopenia and RTNS-Based Intervention
Cachexia Sarcopenia Muscle. 2024 Oct;15(5):2175–2186 (IF = 9.4)
Research Objective:
To identify potential biomarkers associated with sarcopenia in older adults and to uncover key molecular changes in response to resistance training and nutritional support (RTNS).
Study Cohort:
45 patients with sarcopenia and 47 age-matched controls (≥65 years).
Key Findings:
1. Twelve metabolites showed significant differences between the sarcopenia group and controls, including isoleucine, carnitine, 1-methylhistamine/3-methylhistamine, creatinine, carnosine, ureidopropionate, uric acid, PC (18:2/20:0), PC (20:2/18:0), PC (18:1/20:1), PI 32:1, and PI 34:3.
2. Five metabolites were identified as potential biomarkers responsive to RTNS intervention: 1-methylhistamine/3-methylhistamine, carnosine, PC (18:2/20:0), PI 32:1, and PI 34:3. These metabolites may play critical roles in the therapeutic response to exercise and nutritional interventions in sarcopenia.
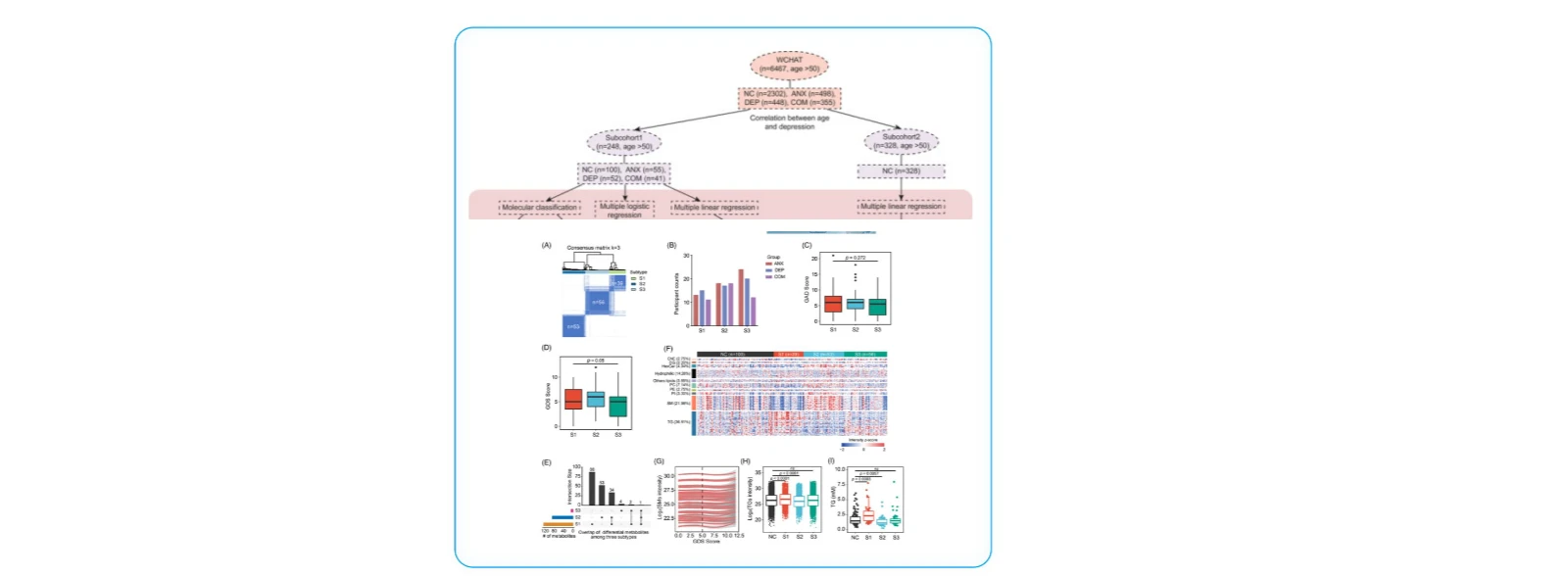
(2)Systemic Metabolic Profiling and Subtyping of Psychiatric Disorders
MedComm (2020). 2022 Sep 30;3(4):e165 (IF = 10.7)
Research Objective:
To investigate shared and distinct metabolic signatures in individuals with anxiety, depression, and comorbid depression.
Study Cohort:
The primary population included older adults (>50 years).
Cohort 1: 100 healthy participants, 55 with anxiety, 52 with depression, and 41 with comorbid conditions.
Cohort 2: 328 healthy individuals.
Additional data: Mental health assessments from 6,467 participants in the WCHAT cohort.
Key Findings:
1. Unsupervised lipidomic clustering stratified psychiatric patients into three metabolic subtypes (S1, S2, S3). S1 exhibited elevated triglycerides and reduced sphingomyelins, while S2 showed the opposite pattern. S3 had a metabolomic profile similar to healthy controls. Individuals in S1 and S2 had poorer quality of life and were more prone to sleep and cognitive impairments compared to S3.
2. Analysis of the WCHAT cohort (n = 6,467) confirmed an age-related increase in depression prevalence. Seventeen depression-associated metabolites showed significant correlations with age, validated in the independent Cohort 2.
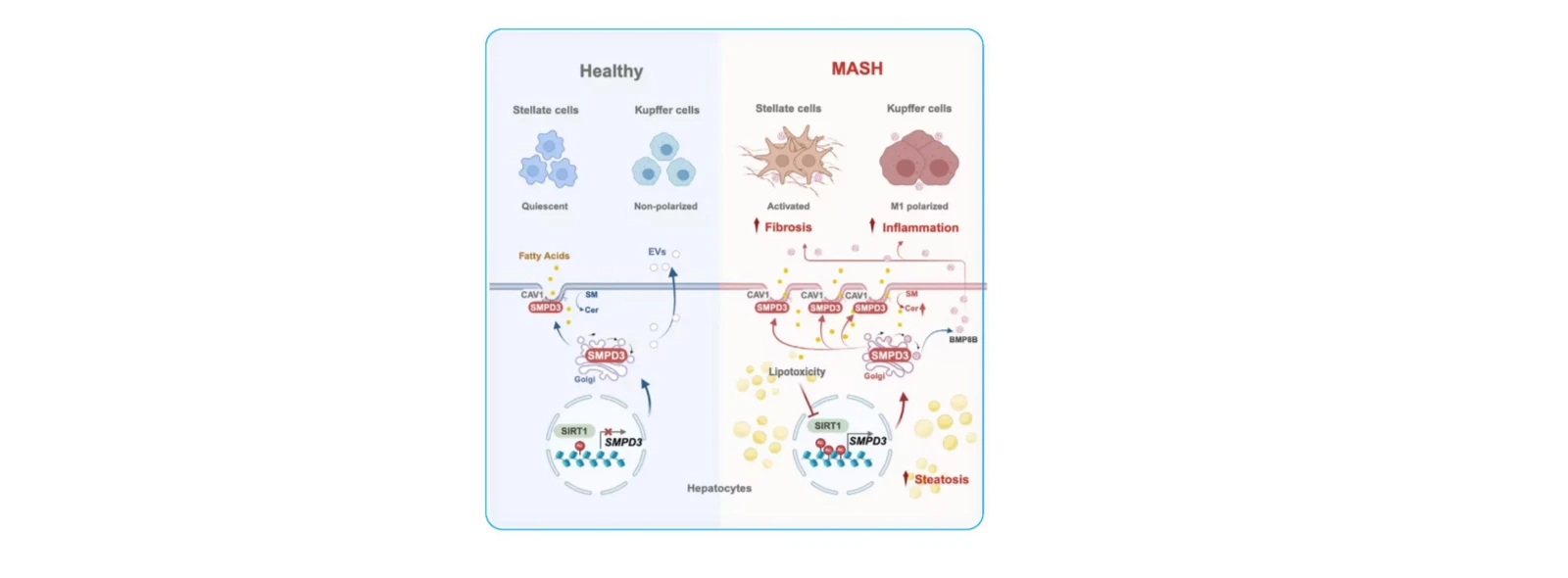
(3) Molecular Mechanisms of Steatohepatitis
Cell Metab. 2025 May 6;37(5):1119-1136.e13. (IF=27.7)
Objective: To investigate the molecular mechanisms by which SMPD3 influences the progression of Metabolic dysfunction-Associated Steatohepatitis (MASH).
Study Cohorts: Multiple MASLD cohorts.
Key Findings:
1. SMPD3 identified as key driver of hepatic ceramide accumulation: Sphingomyelin phosphodiesterase 3 (SMPD3) drives hepatic ceramide accumulation through enhanced hydrolysis of plasma membrane sphingomyelin. Hepatocyte-specific Smpd3 knockout or pharmacological inhibition of SMPD3 protein alleviated MASH, while SMPD3 reintroduction reversed this therapeutic effect.
2. Disrupted membrane sphingolipid metabolism is a core feature of MASH progression: Dysregulation of hepatocyte membrane sphingolipid metabolism represents a core metabolic signature during progression from Metabolic dysfunction-Associated Steatotic Liver (MASL) to MASH, with caveolar region-localized SMPD3 serving as the primary driver enzyme.
3. SMPD3 exacerbates MASH through dual pathways: By altering the sphingomyelin-ceramide metabolic balance in caveolar regions, SMPD3 accelerates lipid uptake and promotes steatosis. Concurrently, it enhances the release of pro-inflammatory and pro-fibrotic extracellular vesicles from hepatocytes, culminating in MASH progression.